My Research
See my current publications here
An Electrochemical Aptamer-Based Biosensor
I am currently based in the Smart Materials and Surfaces Group in the School of chemistry at the University of New South Wales, working with Scientia Prof. Justin Gooding. Our team is currently developing next generation electrochemical aptamer-based biosensors for Nutromics.
-01.png)
Nutromics are developing the first real-time, high-frequency molecular sensing technology that is both: (1) selective enough to work in situ in the living body and (2) independent of the chemical reactivity of its targets, thus rendering it is generalizable. Using this approach, they have demonstrated seconds or even sub-second resolve measurement of multiple drugs, metabolites and biomarkers in the plasma (veins), cerebrospinal fluid (brains) and interstitial fluid (subcutaneous space) of live rats.
I’m sorry, but the exact involvement in this project cannot be discussed at current time as it is currently undergoing the patent process which has not been finalized yet; however, I have linked some references at the bottom which might be of interest.
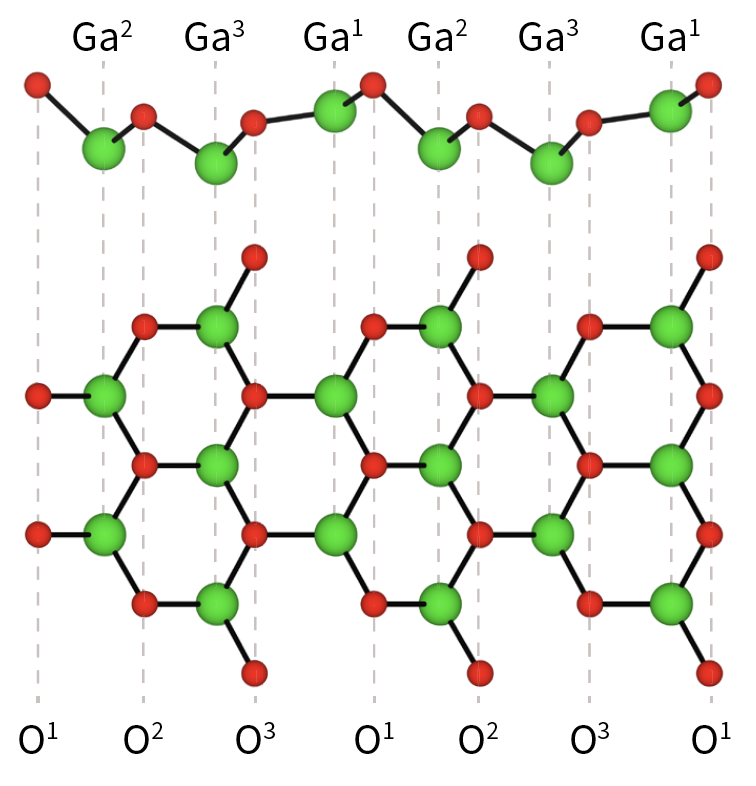
Covalently-Bound Organic Modification of Transparent Metal Oxides
As a PhD student in the School of Physical and Chemical Sciences at the University of Canterbury, I worked with Prof. Alison Downard, Prof. Martin Allen, and Prof. Roger Reeves exploring the physical, chemical, and electronic properties and device applications of metal oxide semiconductors.

The main areas of my project involved:
- Electrochemical and spontaneous modification of metal oxide surfaces
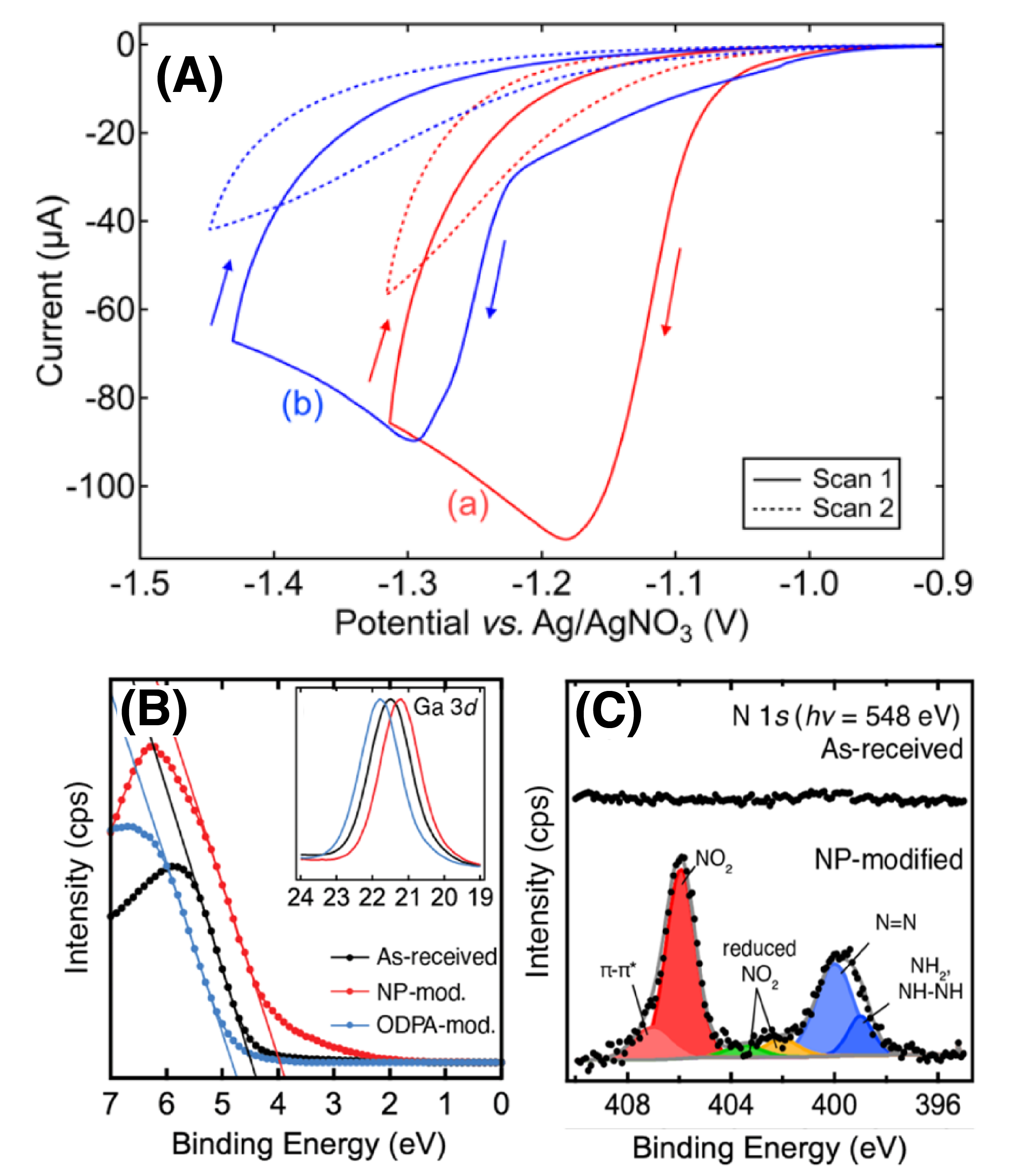
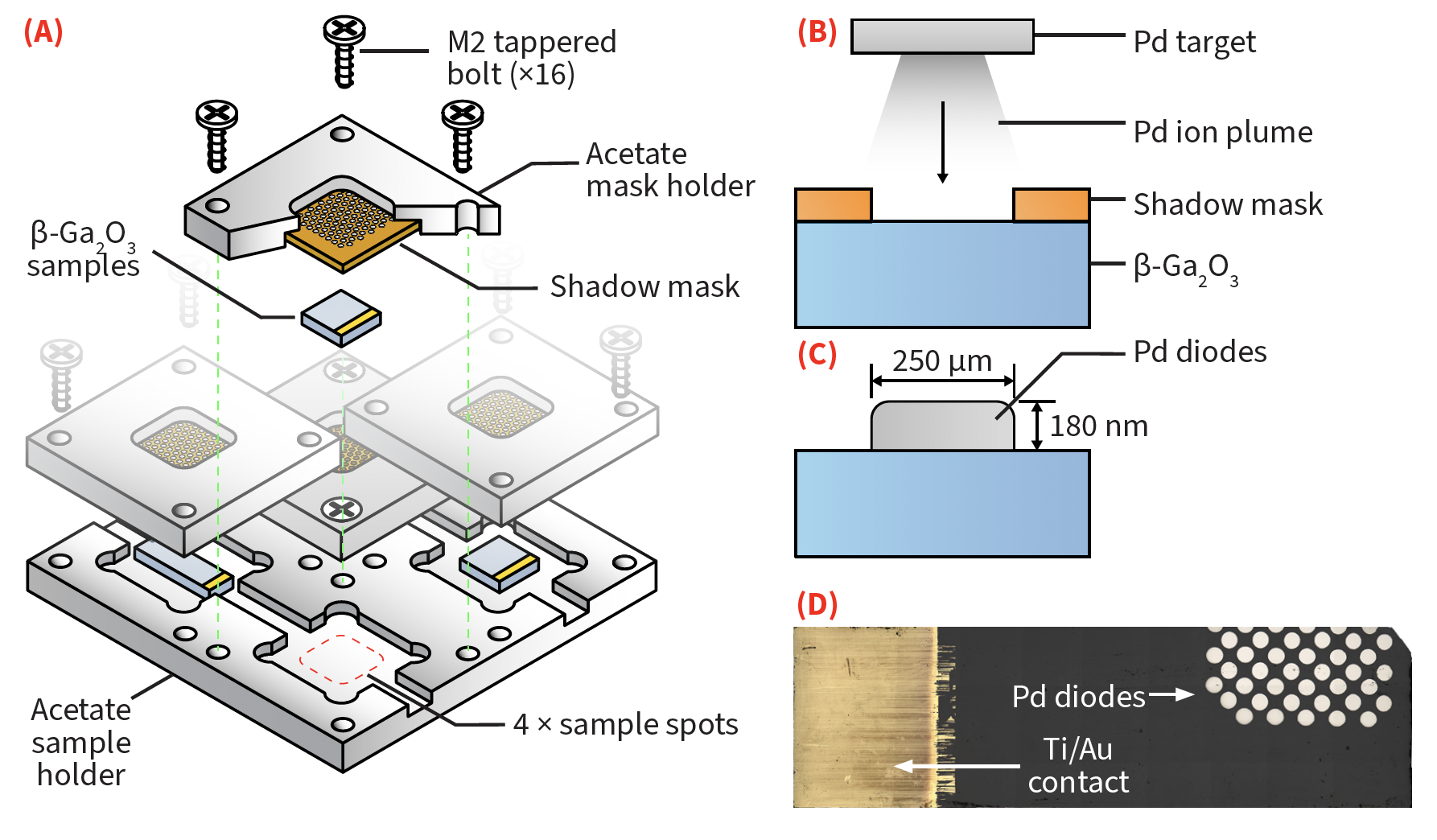
- Nanofabrication of Schotty diodes
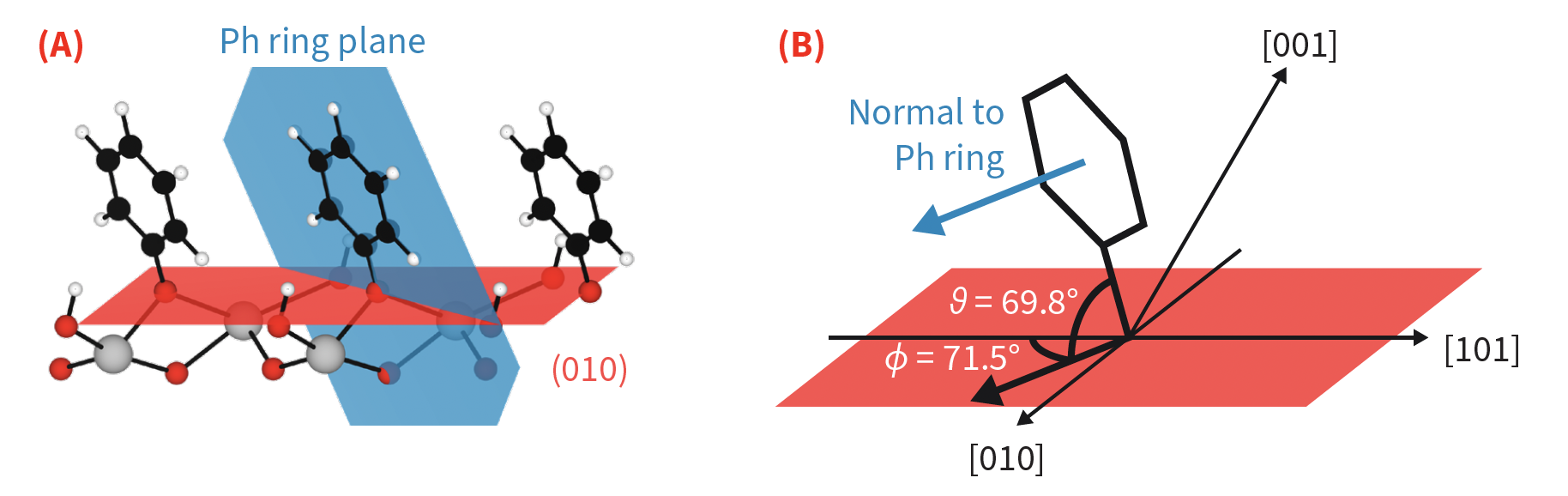
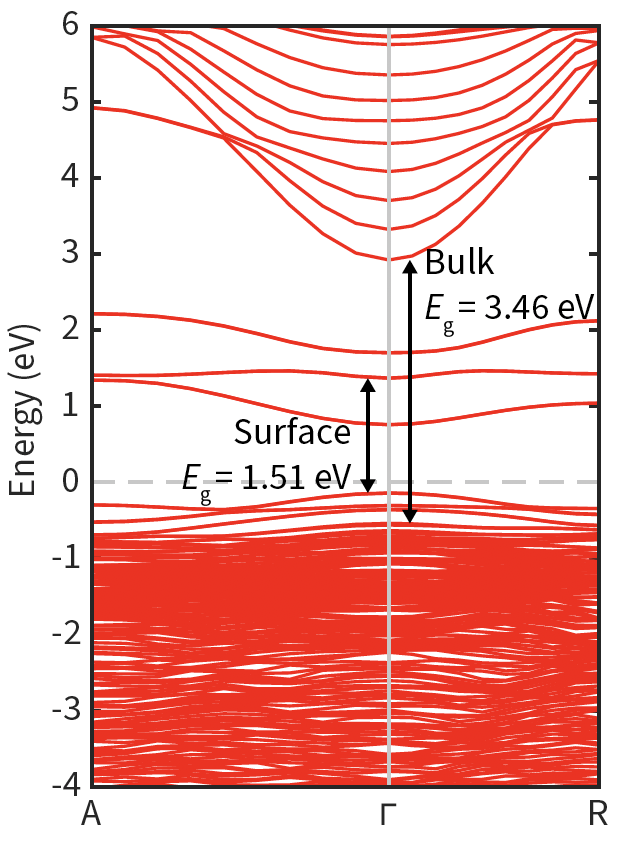
- Computational analysis of the metal oxide surfaces before and after the attachement of aryl groups
Link to my PhD thesis. Currently this is under embargo, however if you are interested I can send through a copy.
Novel Coatings for Improving the Corrosion Resistance of Mg Biomedical Implants
My Honours thesis project, under the supervision of Prof. Alsion Downard and Ass. Prof. Mark Staiger, focused on the development of a biocompatible layer to decrease the corrosion properties of the biomedical magnesium.
A link to my thesis can be found here: Novel Coatings for Improving the Corrosion Resistance of Mg Biomedical Implants
Ultra-High Temperature Electrolysis to Produce Titanium
The use of molten oxide electrolysis to produce metals has been proven more sustainable and environmentally friendly than the common, carbon-intensive, traditional metallurgical processes. The potential to reduce emissions to the environment increases if large-scale waste materials are used as feedstock for this process. It has been shown that the electrochemical recovery of metals from molten TiO2−SiO2−Al2O3−MgO−CaO slag, a by-product of some ironmaking processes, is feasible, although the process had very low faradaic efficiency. Moreover, Ti-bearing slag has been proposed as a substitute for rutile as the feedstock for the titanium industry. However, a more extensive understanding of the electrical properties of this complex oxide system in its molten state is paramount in the design of industrial electrochemical cells.
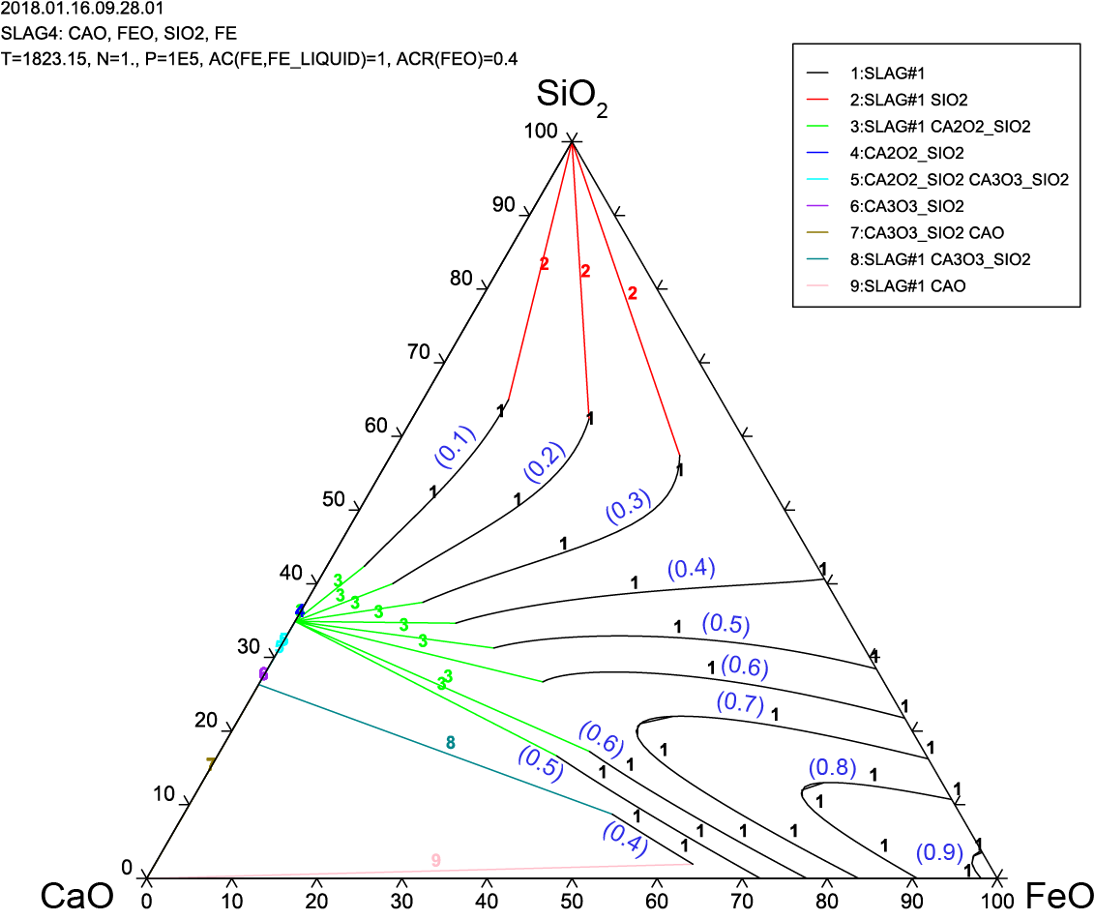
Project Goals:
- Use slag by product while it is still in its molten state (at 1750 K)
- Lower costs
- Better thermodynamics and kinetics
- Investigate various parameters
- Electrolytes (if any)
- Electrode materials
- Additives
- Commercial pathway
- Produce fine powder for 3D printing Conducted thermodynamic calculates relating to the electrolysis of slag and evaluate Thermo-Calc as a viable software package for this.